In today’s fast-evolving pharmaceutical landscape, understanding complex biomolecules is paramount. The intricacies of protein structure and function can often be the linchpin determining the success of a new therapeutic or the precision of a diagnostic tool. This is where LC-MS/MS, or Liquid Chromatography-Tandem Mass Spectrometry, steps in as a revolutionary tool. For industry professionals well-acquainted with LC-MS, the technology’s possibilities are transformative. Liquid Chromatography-Tandem Mass Spectrometry combines two potent techniques: Liquid Chromatography (LC) separates
EXTRACTABLES AND LEACHABLES – A RISK-BASED APPROACH TO IDENTIFY AND QUANTIFY THE UNKNOWNS USING ORBITRAP LC-MS/MS, GC-MS, AND ICP-MS It is an undeniable fact that plastic has been woven into the fabric of modern pharmaceutical and biotech manufacturing processes, owing to its affordability, versatility, and functionality. Yet, these benefits are not without their drawbacks. Given that plastic polymers rely on additives to achieve their necessary properties, the migration of these substances into drug products is a common
Pharmaceutical drug development and approval necessitate intricate and demanding procedures, which culminate in the presentation of a New Drug Application (NDA). A key component of this application is the Chemistry, Manufacturing, and Controls (CMC) section. The preparation and submission of this section, which has to adhere strictly to FDA regulatory requirements and the Common Technical Document (eCTD) format, can be regarded as the core of any pharmaceutical drug application. The CMC serves multiple functions that can determine the overall success of
At Synergy Bioscience, we understand that pharmaceutical and bio-pharmaceutical development is a complex process requiring expertise in various fields. Our comprehensive formulation development services cater to a wide range of molecules, including small chemical molecules and large protein molecules such as enzymes, monoclonal antibodies, growth factors, complex glycoproteins, and novel recombinant constructs. Whether you need a standalone service or a comprehensive development program, our team of experts is dedicated to providing tailored solutions
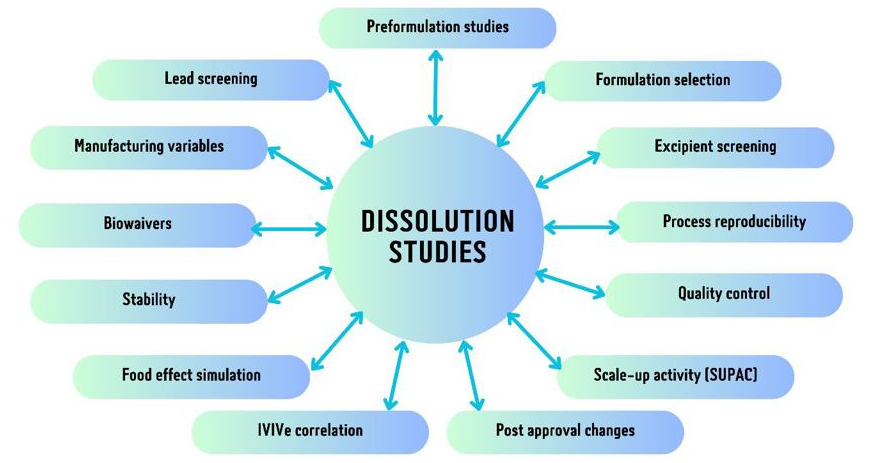
Synergy Bioscience – Mastering Pharmaceutical Dissolution Testing Pharmaceutical dissolution testing stands as a cornerstone in evaluating drug product quality throughout the development and manufacturing stages. This pivotal test gauges the release of a drug’s active substance within a controlled laboratory setting designed to emulate in-vivo conditions. It serves as an indispensable parameter for determining drug product efficacy and safety. In this article, we will explore the importance of dissolution testing and the reasons why Synergy Bioscience emerges as the premier choice
As a vital component of patient safety, endotoxin testing is essential for medical devices, injectables, and their raw materials. Endotoxins, small hydrophobic molecules found in the outer membrane of Gram-negative bacteria, can be released when bacteria die, triggering toxic reactions. Unfortunately, endotoxin testing is sometimes neglected, with many manufacturers relying heavily on sterility testing. However, sterility testing only detects live microorganisms and may fail to identify released endotoxins during the sterilization process validation. The consequences of undetected
Introduction The pharmaceutical industry is a highly regulated sector, with a primary focus on ensuring the safety, quality, and efficacy of medicinal products. One critical aspect of this process is the identification and quantification of extractables and leachables (E&L) in drug products and their packaging. These studies are essential for minimizing potential health risks and maintaining compliance with regulatory guidelines. Synergy Bioscience, a leader in the field, offers comprehensive solutions for E&L analysis that help manufacturers guarantee product safety and meet
Container Closure Integrity Testing (CCIT) is critical to ensuring the safety and efficacy of pharmaceutical, biological, and vaccine products. The adequacy of container closure systems to maintain a sterile barrier against potential contaminants such as microorganisms, reactive gases, and other substances is evaluated through this assay. Synergy Bioscience is a company that specializes in providing state-of-the-art CCIT capabilities with uniquely qualified scientists to exceed client expectations. Proper container closure systems are essential to maintain product and consumer
Synergy Bioscience is at the forefront of harnessing the full potential of orbitrap mass spectrometry, providing cutting-edge solutions for a wide range of applications. This technology has become essential for the chemical characterization of pharmaceutical and biological products, accelerating development pipelines, and improving the quality and efficiency of life-changing therapeutics. In this blog post, we will explore various application areas where Synergy Bioscience’s mass spectrometry solutions shine. 1: Proteomics Applications Synergy Bioscience’s mass spectrometry solutions enable the characterization
Pharmaceutical extractables and leachables testing is a crucial aspect of ensuring drug safety and quality. The process involves assessing potential interactions between primary container-closure systems, packaging components, and the drug dosage form. In this blog, we’ll discuss the importance of such testing and highlight the technical capabilities of Synergy Bioscience, a leader in the field. Why Extractables and Leachables Testing Matters: Extractable are chemical species that
- 1
- 2